Introduction
Probiotics are defined as “live microorganisms which when administered in adequate amounts confer health benefits to the host” by the Food and Agriculture Organization and World Health Organization (Joint, 2002). It has been known that probiotics beneficially impact all over the human body including prevention and alleviation of infectious diseases, immune-related disorders, metabolic diseases, atherosclerosis, cancers, and neurological disorders (Bomba et al., 2012; Kwon et al., 2010; Liu et al., 2018; Suganya et al., 2020).
Probiotics have been consumed in the form of tablets or capsules manufactured through the drying process (Yeo et al., 2018). Lyophilization is a commonly used method to increase microorganism viability during extended preservation in the industry field (Gwak et al., 2015; Lodato et al., 1999). In the process of lyophilization, however, probiotic cells are exposed to conditions with extreme stress and damage, which gives rise to the loss of viability by osmotic shock and the disruption of cell membrane structure by dehydration and oxidation damage (De Wouters et al., 2015; Fiocco et al., 2019; Lodato et al., 1999; Ramírez-García et al., 2018). When those damaged probiotic cells were consumed orally, their probiotic activity would be compromised because of the deterioration in survival in the gastrointestinal tract and adherence to intestinal epithelial cells (Arellano et al., 2021).
To overcome this limitation, rehydration has been introduced as a method to recover normal probiotic function from inactivated, lyophilized form of bacteria (De Valdez et al., 1985). Rehydration is a critical step in the recovery of lyophilized bacteria. Bacteria was damaged during the freeze dry. Damaged cell may be repaired and regain moral function if it is rehydrated with optimized condition considered composition of the rehydrating solution, osmolarity, etc. Lyophilized bacteria cells could experience another osmotic shock during the rehydration process, which could also decrease the cell viability in the gastrointestinal tract (Fiocco et al., 2019; Leach et al., 1959). Several cryoprotective agents and additives for the process of lyophilization and rehydration have been recommended to minimize cell damage and to improve its stability including proteins, amino acids, sugars, and carbohydrates (Bron et al., 2011; Hubálek et al., 2003).
Several microbial factors such as surface proteins, cell hydrophobicity, and electric charge are known to be related to enhance these probiotic characteristics (De Wouters et al., 2015). Especially, zeta-potential is a major factor which can affect electrostatic interactions in particle dispersions, and it is important for understanding the stability of colloidal dispersions such as probiotic solution. The zeta-potential of microbial cell is developed at the interface between the cell surface composition (solid) and the surrounding medium (liquid) (De Wouters et al., 2015). Since Ramírez-García (2018) showed that a higher absolute value of zeta-potential generates more stable the dispersion, the zeta-potential value may serve as a novel parameter to predict cell viability affected by cell membrane damage and its permeability (Halder et al., 2015).
In this study, we have named mixture “Zeta-bio®” technology, which proposed to be freeze-dried probiotics with a special activated substance-based formulation for support the re-activation of a probiotics in water and utilizing this technology, we tried to develop optimal rehydration conditions by adding special activated substance to commercial probiotic mixture (Lc. rhamnosus HN001, Lactobacillushelviticus R-52, Bifidobacterium animalis subsp. lactis HN019 and B. bifidum R0071) (Arellano et al., 2021). First, changes of zeta-potential by 14 putative activators were measured in each probiotic strain, and the competency was evaluated by simulated stomach-duodenum passage (SSDP) assay and cell adhesion assay with the mixture and its representative strain, Lacticaseibacillus rhamnosus HN001.
Materials and Methods
Lc. rhamnosus HN001 and L. helviticus R-52 were cultured in MRS broth (BD, Franklin Lakes, NJ) at 37°C for 18~20 h. B. lactis HN019 and B. bifidum R0071 were cultured in BL broth (KisanBio Inc., Seoul, South Korea) at 37°C under anaerobic condition for 18h. All strains were provided by COSMAX NBT Inc. (Seongnam, South Korea).
The candidate chemicals were selected based on “Food Additive Status List” provided by the U. S. Food & Drug Administration (Silver Spring, MA) (Table 1). Each ingredient was dissolved in pH 2.5 HPLC pure water and lyophilized strains were rehydrated with HPLC pure water. To measure the zeta-potential, 1 mL of the ingredient solution (0.3 M) and 1 mL of test strains (1×109 CFU/mL) were added to 8 mL of pH 2.5 HPLC pure water, at the final concentration of 1×108 CFU/mL of bacteria and 0.03 M of the supplement. The sample was transferred to a folded capillary zeta cell (DTS1060C, Malvern Panalytical Inc., Westborough, MA) and measured at 25°C using Zetasizer Nano ZEN 3600 (Malvern Panalytical Inc.).
SSDP assay was conducted according to Ji et al. (2013) with minor modifications. Freeze-dried Lc. rhamnosus HN001 was separately mixed with each of the amino acid, threonine, phenylalanine, lysine, and histidine, at a concentration of 0.03M, resulting in a final concentration of 1×109 CFU/mL. After 5 min rehydration, 1 mL of each sample was added with 9 mL of acidic phosphate buffer saline (PBS, pH 3.0), and then incubated at 37°C for 1 h. Subsequently, 17 mL of duodenum juice (NaHCO3: 6.4 g/L, KCl: 0.239 g/L and NaCl: 1.28 g/L, pH 7.4) and 4 mL of bile salts solution (10% oxgall, BD) were added and incubated for additional 2 h. To check the survival rate, samples were collected at each point of exposure (0h for unexposed, 1h for acid-exposed and 3h for acid and duodenum juice-exposed) and counted using serial dilution with MRS (BD) under anaerobic condition at 37°C for 48 h. With 4-strain bacterial mixture, the SSDP assay was conducted with histidine supplementation at concentrations from 0.01 M to 0.05 M. Samples collected at each time point was cultured using MRS agar and BL agar (BD, USA) under anaerobic conditions at 37°C for 48 h.
The lyophilized probiotic mixture as was rehydrated with 0.03 M histidine-PBS solution or normal PBS solution for 5 min to verify the effect of histidine on the stability of bacteria. Samples were collected at 0, 15, 30, 45 and 60 min after rehydration, and the viability was checked by serial dilution using MRS agar incubated under anaerobic conditions at 37°C for 48 h.
The human intestinal epithelial cells (HT-29, distributed by the Korean Cell Line Bank) were grown and maintained in RPMI 1640 (Corning, NY) containing 10% fetal bovine serum (Gibco, Thermo Fishcer Scientific, Waltham, MA) and 1% antibiotics (antibiotic-antimycotic, Gibco) at 36.5°C and 5% CO2 condition. Cell adhesion test was performed according to Botes (2008) and Kavanaugh (2013) with modification. Briefly, eukaryotic cells were seeded into 12-well plate (Corning) at 1×106/well and incubated overnight. Two hours prior to assay, cells were washed with PBS to remove antibiotics and then media was substituted for serum-free RPMI 1640. The lyophilized bacterial strain and the 4-strain bacterial mixture were rehydrated in 10 mL of 0.03M histidine for 5 min. HT-29 monolayer was treated with the bacteria suspension at the ratio of 100:1 (Bacteria: cell) and incubated at 37°C for 40 min in an anaerobic chamber (Whitley DG250 anaerobic workstation, Don Whitley Scientific, Bingley, United Kingdom). After incubation, cells were washed to remove excessive bacteria, detached with 500 μL of trypsin-EDTA solution (Gibco), and then subsequently added with additional 500 μL of RPMI 1640. The cell suspension and the original inoculum were serially diluted, and bacterial count was carried out in MRS or BL agar (BD, USA) incubated under anaerobic condition at 37°C for 48 h. The percentage of adherent bacteria was calculated, and relative changes were expressed as fold changes compared to the non-reactivated control group.
All data were presented as mean ± S.D. (n=3). Statistical analyses were performed using GraphPad Prism version 9.3.1 (GraphPad, La Jolly, CA) and Microsoft Excel. Comparisons of experimental groups were performed using ordinary one-way analysis of variance (ANOVA) with proper post hoc analysis with α = 0.05. P values < 0.05 were considered as statistically significant.
Results
The zeta-potential of 4 rehydrated bacterial powder was measured with 14 candidate materials each (Table 1) to select ingredients which would improve dispersion stability of probiotic bacteria. Addition of L-histidine, L-tyrosine, L- threonine, and L-phenylalanine increased negative charge values of Lc. rhamnosus HN001 zeta-potential (Fig. 1A). L-histidine, L-tryptophan, L-threonine, L-lysine, and L-phenylalanine gave higher negative charge value than control sample with B. lactis HN019 (Fig. 1B). The zeta-potential of B. bifidum R0071 was altered by adding L-histidine, L-tryptophan, L-threonine, L-lysine, and L-phenylalanine (Fig. 1C). For L. helviticus R-52 strain, addition of L-histidine significantly changed the zeta-potential to have extremely negative charge value (Fig. 1D).
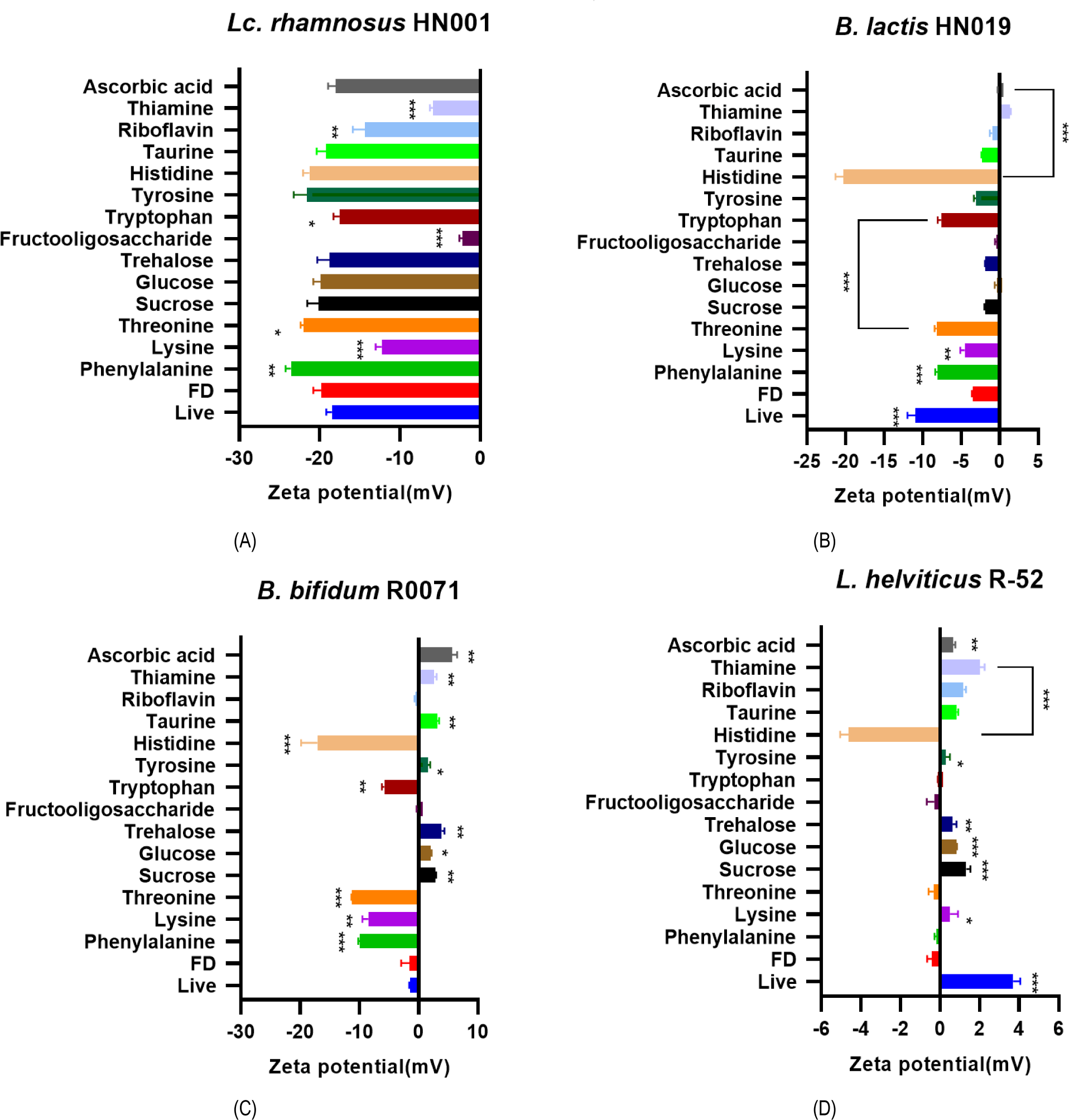
Based on the zeta-potential assay result, 4 potent ingredients were selected as additive candidates for the rehydration process: L-histidine, L-threonine, L-lysine, and L-phenylalanine. To validate their effect on probiotic properties, the survivability in the gastrointestinal tract was examined by SSDP assay with the 4-strain mixture and its representative strain, Lc. rhamnosus HN001. Lyophilized powder of Lc. rhamnosus HN001 showed significantly deteriorated survival rate compared to the live control group. Among 4 amino acid (AA) candidates, L-phenylalanine and L-histidine significantly increased the survival rate, and L-histidine showed higher potency than L-phenylalanine (Table 2). To optimize L-histidine concentration, the 4-probiotic strain mixture powder was rehydrated with different concentrations of L-histidine from 0.01 M to 0.05 M. L-histidine supplementation notably increased the bacteria survival rate (Table 3), and the highest survival rate was observed at the concentration of 0.03 M.
To evaluate the effect of L-histidine supplementation on the probiotic strain stability under extended rehydration time, bacterial cell viability was examined for 1 h was examined. With supplementation of L-histidine at optimal dose, 0.03 M, cell viability of the rehydrated probiotic mixture was maintained stably (105.9% at T=60 min, Table 4), while the control group without L-histidine showed relatively reduced survival rate (54.4% at T=60 min).
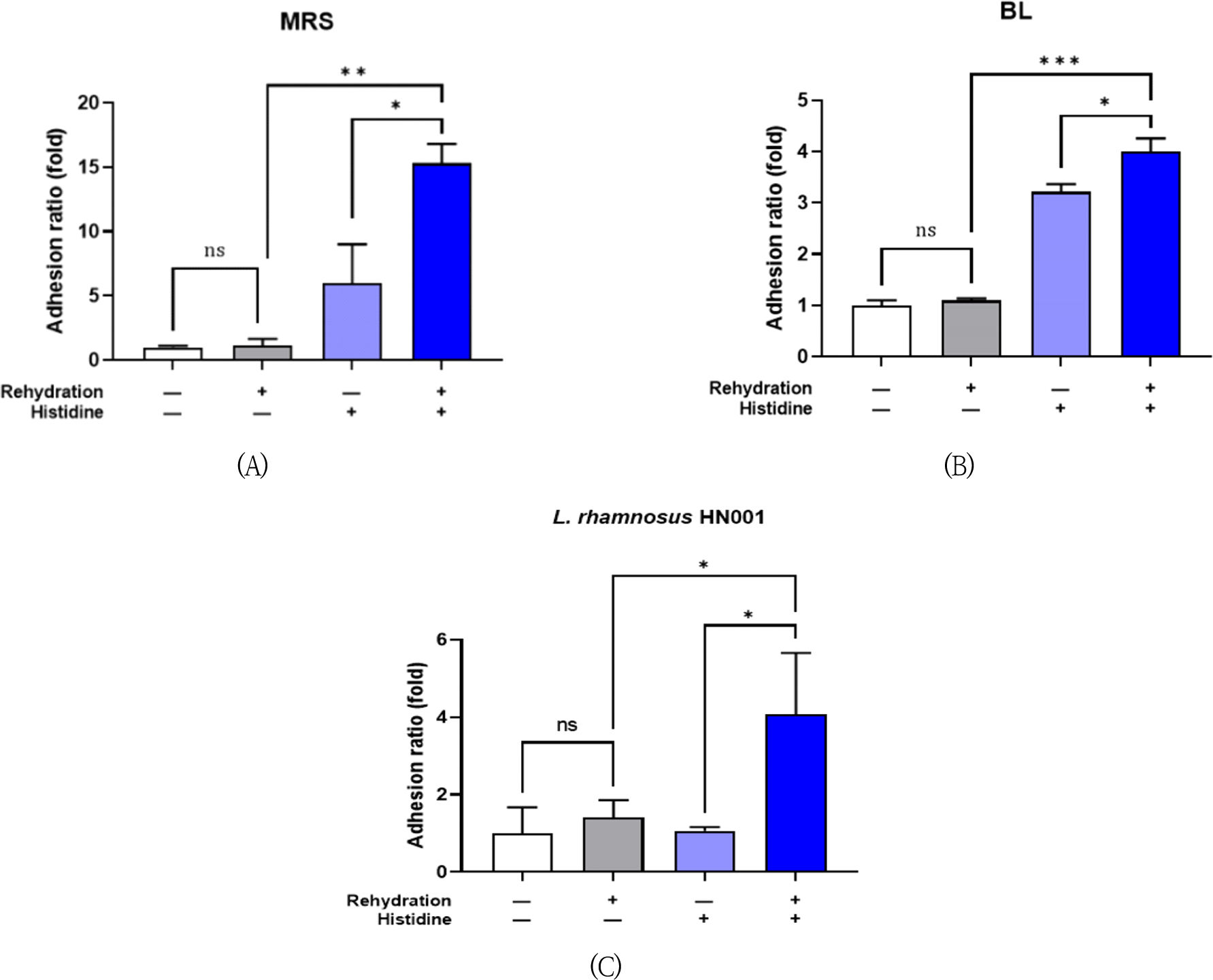
Next, bacterial cell adherence capability was examined by adhesion assay using human intestinal epithelial cell line. The lyophilized bacteria, 4 strains mixture or Lc. rhamnosus HN001, were rehydrated with 0.03 M L-histidine for 5 min and then treated to the enterocytes. L-histidine supplementation significantly increased the adhesion ratio 15.3-fold and 4.00-fold compared to each non-supplemented control in MRS agar (Fig. 2A) and BL agar (Fig. 2B), respectively. The adhesion ratio of Lc. rhamnosus HN001 was also increased 4.07-fold by histidine rehydration (Fig. 2C).
Discussion
Previous research indicates the significance of bacterial viability for its efficacy. Nevertheless, the harsh gastrointestinal environment poses a considerable challenge to probiotics when orally ingested, primarily attributed to the presence of intestinal bile and stomach acid. Most of commercialized probiotics are lyophilized to be stably transported and to extend shelf-life by reducing water activity. On the other hand, the process is still stressful enough to substantially deteriorate bacteria viability since it damages the integrity of cell membrane as well (Govander et al., 2014). Rehydration is vital for recovering lyophilized bacteria, enabling damaged cells to potentially repair and regain normal function under optimized conditions. To overcome this problem, lyophilized bacteria must have similar viability as much as live bacteria (Schwab et al., 2007). It needs to be well dissolved lyophilized bacteria for similar stability of live bacteria.
Cell’s zeta potential will be determined by the cell surface composition and the properties of the surrounding medium (Soon et al., 2011). In this study, FDA-approved 14 ingredients were tested for their modulating effect on zeta-potential value of lyophilized bacterial strains (Fig. 1). Halder (2015) showed that decrease zeta-potential absolute value was related with the increase of bacterial membrane permeability, which leads to cell death. Previous studies showed that giving an appropriate negative charge of zeta-potential to the probiotics cell surface can reactive probiotic cells, recover damaged cells, and improve their viability (Arellano et al., 2021; Cowan et al., 1992). It was found that 4 AA, L-histidine, L-threonine, L-lysine, and L-phenylalanine enhanced zeta-potential value, and these chemicals could be utilized as potential additives for enhanced rehydration condition. AA are important for bacteria growth, biofilm formation, and dispersal (Idress et al., 2020). Bacterial peptidoglycan is an important polymer serving structural role in the bacterial cell wall, which is related with the strength of the wall. This polymer is composed of sugar and AA, and the strength and the elasticity of the wall show positive relation with AA supplementation. In our study, selected 4 AA for the lyophilized bacteria increased the stability of rehydrated bacteria, and this could be attributed to the strengthened function of bacterial cell walls.
Survivability of probiotics under gastrointestinal stress is also mainly related to its beneficial effect on the host. We evaluated the effect of 4 AA supplementation on the survival rates of the probiotic mixture using SSDP assay, a representative in vitro experiment method mimicking the environment of the stomach and small intestine (Mathara et al., 2008). Among those 4 AA, L-histidine at the concentration of 0.03 M showed the most enhanced survivability (Table 2 and Table 3). In addition, L-histidine supplementation improved bacteria viability during rehydration for extended time (Table 4). Several AA metabolisms such as histidine, glutamine, aspartic acid and arginine could be utilized to increase bacterial acid resistance, and histidine metabolism could be coupled with proton-consuming reactions in strain-dependent way (Hall et al., 2019; Trip et al., 2012). It is also possible that L-histidine is more effective for restoration of rehydrate bacteria which may experience damage in cell integrity during lyophilization process.
It is also important to verify adhesion ability of probiotic cells to intestinal mucosa which influence the interaction between probiotics and the host system. The adhesion ability is related with bacterial cell surface properties such as hydrophobicity, extracellular polymers (exopolysaccharides, adhesins), and the electric charge (Alander et al., 1999). In our study, rehydration with 0.03 M L-histidine significantly augmented the attachment to intestinal epithelial cell (Fig. 2).
L-histidine is considered an essential amino acid for infants and is related to growth in infants including weight gain when it is insufficient (Snyderman et al., 1963). It is not synthesized endogenously at necessary concentration of physical demand. Histidine is also used to make histamine, a common cause of allergy reaction, in human body (Holeček et al., 2020; Thalaker-Mercer et al., 2020). However, L-histidine is “generally regarded as safe” (GRAS) by the FDA and many studies suggested its use as food additives (US Food and Drug Administration, 2020). Gibbs (2020) reported effect of long-term histidine administration on young children and adult with atopic dermatitis (AD) and suggested that histidine is safe and convenient supplement for management of AD in both (young children and adult). L-histidine is known as an osmoprotectant, help enhance the resistance of a strain under unbalanced osmotic conditions (Bougouffa et al., 2014).
In conclusion, addition of 0.03M L-histidine mixture, designated “Zeta-bio®” can increase the viability of freeze-drying cells and can improve stability in in vitro gastric and duodenum condition and can improve effect of cell adherence function. Therefore, this study shows possibility that histidine is restored damage of lyophilization and ameliorated survivability for probiotics commercialization.







